Chlorine Dioxide as Alternative Disinfectantin Drinking Wate
| 論文類型 | 技術與工程 | 發表日期 | 2005-10-01 |
| 作者 | Via,Friuli | ||
| 摘要 | A review with references on the applications of Chlorine Dioxide (ClO2) in drinking water treatment, based on a long and established experience in the European Countries.The main source for drinking water supplies is surface water and the relevant micro | ||
Chlorine Dioxide as Alternative
Disinfectantin Drinking Water Treatment
Alessandro Francesconi Antonella Longhi
Water Treatment Division, INDUSTRIE CHIMICHE CAFFARO SpA, (SNIA BPD Group),
Via Friuli, 55 - 20031 Cesano Maderno (MI) - Italy
Abstract A review with references on the applications of Chlorine Dioxide (ClO2) in drinking water treatment, based on a long and established experience in the European Countries.
The main source for drinking water supplies is surface water and the relevant microbial contamination may cause diseases (e.g. cholera, typhus, dysentery), a big problem not only in developing Countries, but also in fully industrialised countries such as USA. Therefore it is necessary to disinfect water with a persistent chemical to avoid microbial regrowth and ClO2 is the best chemical suitable for this purpose.
In the drinking water treatment Chlorine Dioxide has been successfully used for many years. It is a very strong oxidising agent and a very active disinfecting agent: these features make it suitable both in the pre-oxidation and in the final disinfection stage of the conventional drinking water treatment.
In the pre-oxidation stage it is able to improve the iron and manganese removal, in the same time reducing the problems of bad tastes and odours and controlling the algae growth in the system. In the final disinfection stage low dosages of ClO2 with short contact times are able to guarantee a constant and reliable control of the microbial quality of the water supplied to the community.
Chlorine Dioxide does not produce halogenated by-products as well as other chlorine compounds. For this reason and considering the arising sensitivity and concerns about the toxicity of the disinfection by-products (DBPs) of the traditional chlorination, Chlorine Dioxide has become widely used, replacing conventional chlorine disinfectants.
Keywords Chlorine dioxide disinfection; disinfection by-products (DBPs) drinking water
Introduction
In the last years the deteriorated quality and the reduced quantity of appropriate sources of drinking water have highlighted the need of advanced and reliable technologies able to ensure the required performance with reasonable costs. "Advanced" in terms of limiting as much as possible the direct and indirect impact on the human health, taking care of the increased scientific knowledge and the raised sensitivity of the people. "Reliable" in terms of possibility to be always used, even when there are not the required ideal conditions of application.
Among the chemical and physical treatments necessary to make water potable and suitable for human consumption, the disinfection stage has been widely studied. Its occurrence has been confirmed essential in a complete drinking water treatment, not only when suggested by the bad microbiological quality of raw water, but in any case, because the water always is the preferred transfer way of very dangerous diseases, such as cholera or typhus. For this reason in 1993 WHO has still recommended that an "efficient disinfection must never be compromised" by any concern about its cost and/or its chemical by-products.
A lot of efforts have been made to optimise the use of conventional chemicals in disinfecting water (mainly chlorine compounds) and to find suitable alternative products. In this lecture Chlorine Dioxide (ClO2), its established use in this field with some laboratory tests and a comparison with the other disinfectants will be summarised.
Chemical features and main applications of chlorine dioxide
At room temperature Chlorine Dioxide (ClO2) is a yellow-orange gas, heavier than the air.
It is very soluble in water (14 g/L at 25 °C) and does not give any reaction of hydrolysis. In water it is present as dissolved gas in a large range of pH (from 6 to 10). Therefore it has a long lifetime in water, resulting very persistent in the treated water. The water solutions of Chlorine Dioxide, normally used for carrying out lab tests, are stable, if stored in closed glasses, at low temperature and dark conditions.
As far as the chemical reactivity is concerned, ClO2 main characteristics are the following:
it does not react with ammonia;
it does not have a chlorinating action on the organic substrate (mainly humic and fulvic acids), with consequent very negligible formation of A.O.X. and T.H.M.;
it destroys phenol compounds;
it is able to oxidise iron and manganese ions;
it does not oxidise bromide ion;
its activity does not depend on pH.
A.O.X. (Halogenated Organic Compounds Adsorbable on Activated Carbon) and T.H.M. (Trihalomethanes - trichloromethane, tribromomethane, dichlorobromomethane, dibromochloromethane) are the reference parameters controlled in drinking water to evaluate the halogenated by-products of the disinfection treatment. The halogenated organic compounds were recognised potentially toxic for the human health in terms of carcinogenic risk associated to their constant assimilation through drinking water.
The Trihalomethanes may act as an indicator for the presence of other chlorination by-products. Control of the four most commonly occuring Trihalomethanes in drinking water should help to reduce levels of other uncharacterized chlorination by-products. In fact IARC has classified trichloromethane in Group 2B as a possible human carcinogen.
Therefore the last Proposal of the European Community Commission for a Council Directive concerning the quality of drinking water sets the following limits:
trichloromethane 40 mg/L
dichlorobromomethane 15 mg/L
ClO2 is not a chlorurating but a very strong oxidising agent, having usually chloride (Cl-) and chlorite (ClO2-) ions as final products according to the following reactions:
ClO2 + 4 H+ + 5 e- è Cl- + 2 H2O (Eo = 1,51 V)
ClO2 + e- è ClO2- (Eo = 0,95 V)
It has to be produced on application site, by means of specific equipment called "generator". In this reactor Chlorine Dioxide is the final product of a chemical reaction, usually based on sodium chlorite (NaClO2). The principal methods to produce Chlorine Dioxide are the following:
1 2 NaClO2 + Cl2 è 2 NaCl + 2 ClO2
2 NaClO + HCl è HClO + NaCl
HClO + HCl + 2 NaClO2 è 2 ClO2 + 2 NaCl + H2O
3 5 NaClO2 + 4 HCl è 4 ClO2 + 5 NaCl + 2 H2O
4
The last one is the most used and results the best choice especially for drinking water treatment, because it guarantees the best conversion to Chlorine Dioxide, limiting as much as possible the formation of by-products.
Chlorine Dioxide is a very active and strong biocide against bacteria, viruses, algae and fungi. The biocidal action is probably due to its reaction with the vital amino acids of the cells: this is possible thanks to the permeability of cell walls to gaseous ClO2.
Considering its above mentioned characteristics, Chlorine Dioxide has been widely used for many years in the following applications:
drinking water treatment;
waste water (both municipal and industrial) final disinfection;
antifouling treatment of industrial cooling water system;
antislime treatment of the process water in the paper industry;
disinfection treatment in food and beverage industry;
In this report we examine closely the application of ClO2 in the drinking water treatment, where there was a big increase of utilisation in the last years, mainly in France and Italy. In these European Countries the concerns about the possible effects on public health by traditional chlorination by-products and the resulting strict regulations forced the replacement of chlorine/sodium hypochlorite with Chlorine Dioxide.
Drinking water treatment by chlorine dioxide
In the required treatment of raw water (mainly surface water) to make it potable, Chlorine Dioxide can be used both as oxidising and disinfecting agent.
The conventional treatment of this kind of water includes the following stages: pre-oxidation, clarification (with addition of inorganic coagulants, iron or aluminium salts), filtration (by sand and/or activated carbon), final disinfection. With reference to its oxidising activity, Chlorine Dioxide can be dosed in the pre-oxidation stage, just before the coagulation/flocculation, and here it gives the following advantages:
oxidation of Fe and Mn ions and consequent better removal of their relevant hydroxides, very insoluble in water;
destabilisation action of the suspended solids and colloids, resulting a better settlement of the flocs during the clarification;
elimination of the organic compounds (i.e. phenols) which may cause bad odours and tastes, giving a better organoleptic quality of the water;
removal of T.H.M. precursors (organic substrate).
In this case the normal dosage range of ClO2 is 0.5 ? 2 mg/L with a reference time of 15 ? 30 minutes. A secondary notable effect is the control of the algae growth alongside the other treatment stages, which allows a more effective final disinfection.
As disinfecting agent Chlorine Dioxide is used in the final disinfection, after the filtration, where, at a dosage of 0.2 ? 0.4 mg/L, it is able to guarantee an efficient and constant elimination of micro-organisms (bacteria, virus, etc.) content of the finished water, giving the following significant benefits:
negligible formation of organic and inorganic halogenated by-products;
as residual, long lifetime in the distribution network (pipes and reservoirs), able to avoid possible troubles caused by the regrowth of micro-organisms.
As for other chlorine compounds, the "Chlorine Dioxide Demand" is a preliminary assessment, very helpful to define the required dosage to be used case by case. It is the amount of ClO2 which reacts with the raw water in a defined time (from 5 to 60 minutes). This value could be useful to carry out the necessary lab tests and then to adjust the actual dosage on field, also depending on the possible change of the quality of the raw water.
Hereunder are listed two interesting examples of laboratory tests which confirm the performance and the results of ClO2 treatment, mainly as far as the removal of some reference bacterial indicators and the formation of halogenated by-products are concerned. The parameters have been determined in accordance with "Standard Methods for the examination of water and waste water - 19th Edition - 1995".
Example 1
This is a specific comparison between sodium hypochlorite and Chlorine Dioxide treatments for final disinfection of surface water (artificial reservoir; pH = 8.8; ClO2 Demand = 0.57 mg/L) with a contact time of 15 min. The dosages have been chosen considering the mentioned ClO2 demand and the necessity to have a residual ClO2 in the treated water.
The results have confirmed the lower formation of A.O.X. and T.H.M. with ClO2 together a better removal of bacteria and no effect on T.O.C. (Total Organic Carbon) for both chemicals.
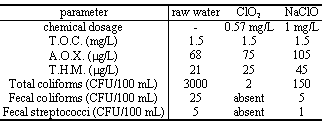
Example 2
This study still compares sodium hypochlorite and ClO2, in the pre-oxidation stage of a surface water (river reservoir; pH = 7.9; ClO2 Demand = 2.05 mg/L). The results have confirmed that ClO2 gives less amount of A.O.X. (- 80 %) and T.H.M. (- 75 %), roughly with the same level of bacteria removal.

Cost/performance comparison with other disinfectants
Considering the European experience of application of ClO2 in drinking water treatment a cost/performance comparison has been done with the other disinfectants, such as chlorine/sodium hypochlorite and ozone, which are the main alternatives of ClO2 for this kind of application.
Even if it is complicated to define a rank which is of general use, in the following tables there are the estimated costs of the specific equipment required for the dosage (including relevant maintenance), the direct cost of the product, and an evaluation of distinct advantages and/or disadvantages of the treatments,.
In fact, depending on the particular conditions of the specific water treatment (i.e. quality of raw water, size of the waterworks, local regulations and/or duties on the products) and the characteristics of the mentioned chemicals, one of them sometimes results the only possible choice.

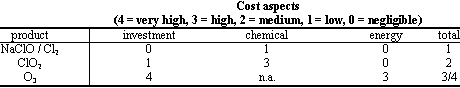
Also the safety aspects in the handling operations connected with the use of these chemicals have always to be considered: for instance in Italy there is a very strict and severe regulation which has limited the use of chlorine gas in the last years.
Conclusions
The chemical characteristics of Chlorine Dioxide make it suitable both in pre-oxidation stage and in the final disinfection stage of the drinking water treatment.
It is performant in all the conditions and it is able to replace the traditional chlorine gas and/or sodium hypochlorite, ensuring a better control of the micro-organism content.
In Europe, Chlorine Dioxide is widely used in drinking water treatment, mainly because of its negligible impact on the halogenated organic by-products formation.
The cost of a disinfection by ClO2 is comparable with the cost of the other used products and its application does not require a specific technological know-how, considering the available equipment present on the market.
The large range of available capacity of Chlorine Dioxide generators allows its application both in big waterworks and in small communities, resulting a reliable and flexible system.
References
(1) Aieta, E.M., Berg, J.D. (1986), A review of Chlorine Dioxide in Drinking Water Treatment, Journal AWWA, 62, 62-72.
(2) Alvarez, M.E., O‘Brien, R.T., (1985), Mechanism of inactivation of Poliovirus by chlorine dioxide and iodine, Appl. Envir. Microbiol., 44, 1064
(3) Bernarde, M.A., et al., (1967), Kinetics and mechanism of bacterial disinfection by chlorine dioxide, Appl. Microbiol., 15, 257
(4) Boardman, G.D., et al., (1979), Alternative Water Disinfectants: an overview, Environmental Engineering, 23-30.
(5) European Commission, (1994) Proposal for a Council Directive concerning the quality of water intended for human consumption, 95/C 131/03 - COM (94) 612 final - 95/0010(SYN)
(6) Flore, J., Ruana, J.F., (1988), Tarragone: problèmes de go-t e d‘odeur, T.S.M. L‘Eau, September, 469-475
(7) Gomella, C., Musquere, P., (1980), La désinfection des eaux par le chlore, l‘ozone et le dioxyde de chlore, XIII Congrès de l‘A.I.D.E., Paris.
(8) Griffini, O. (1996), Disinfection by chlorine dioxide, Proceedings of 2nd Giornata di studio "Disinfection of drinking water" , Brescia (Italy), 49-83.
(9) Hoff, J.C., Geldreich, E.E., (1980), Comparison of the biocidal efficiency of alternative disinfectants, Proceedings AWWA Seminar, Atlanta, Georgia.
(10) Hoff, J.C. (1986), Inactivation of microbial agents by chemical disinfectants, USEPA 600/286/067.
(11) International Agency for Researc on Cancer. Overall evaluations of carcinogenicity: an up-dating of IARC Monographs volumes 1-42. Lyon, 1987
(12) Masschelein, W.J. (1979), Chlorine Dioxide, Chemistry and Environmental impact of Oxichlorine Compounds, Ann Arbor Science Publishers, Ann Arbor, Michigan.
(13) Noss, C.I., et al., (1983), Reactivity of chlorine dioxide with nucleic acids and proteins, Water Chlorination: Environmental Impact Health Effects, Vol.4, Ann Arbor, Michigan.
(14) Olivieri, V.P., et al. (1985), Mode of action of chlorine dioxide on selected viruses, Water Chlorination: Environmental Impact Health Effects, Vol.5, Ann Arbor, Michigan.
(15) Pelizzetti, E., et al., (1994), Disinfection By-Products in Drinking Water Treatments, Scienza e Tecnologia, 76, 701-707.
(16) Walker, G.S., Lee, F.P., Aieta E.M., (1986), Chlorine Dioxide for Taste and Odour Control, AWWA Journal, 3, 84-93.
(17) WHO, (1993), Guidelines for drinking water treatment, Geneva
(18) WHO, (1996), Guidelines for drinking water quality, Geneva
(19) Zavaletta, O., (1992), Chlorine Dioxide Risk Assessment for drinking Water, Second Int. Symposium - Chlorine Dioxide: Drinking Water Issues, May 1992, Houston.
close
www.fun208.com論文搜索
發表時間 至
月熱點論文
論文投稿
很多時候您的文章總是無緣變成鉛字。研究做到關鍵時,試驗有了起色時,是不是想和同行探討一下,工作中有了心得,您是不是很想與人分享,那么不要只是默默工作了,寫下來吧!投稿時,請以附件形式發至 paper@h2o-china.com ,請注明論文投稿。一旦采用,我們會為您增加100枚金幣。








